To Form An Ion A Sodium Atom
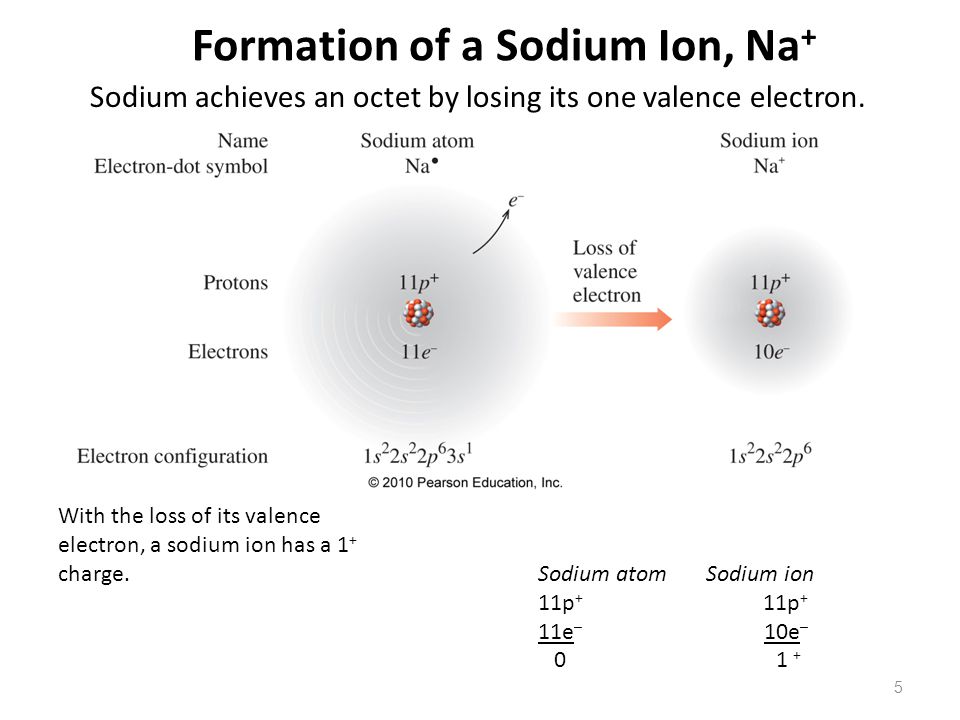
To Form An Ion A Sodium Atom - Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. A neutral sodium atom consists of 11 protons and 11 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons. (i) formation of sodium ion: When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge.
When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: A neutral sodium atom consists of 11 protons and 11 electrons. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons.
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A neutral sodium atom consists of 11 protons and 11 electrons. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: When sodium loses its outermost electron, the atom transforms.
Formation+of+a+Sodium+Ion,+Na+
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A.
Structure Of Sodium Atom Hot Sex Picture
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. A neutral sodium atom consists of 11 protons and 11 electrons. The sodium ion, na +, has the electron configuration with.
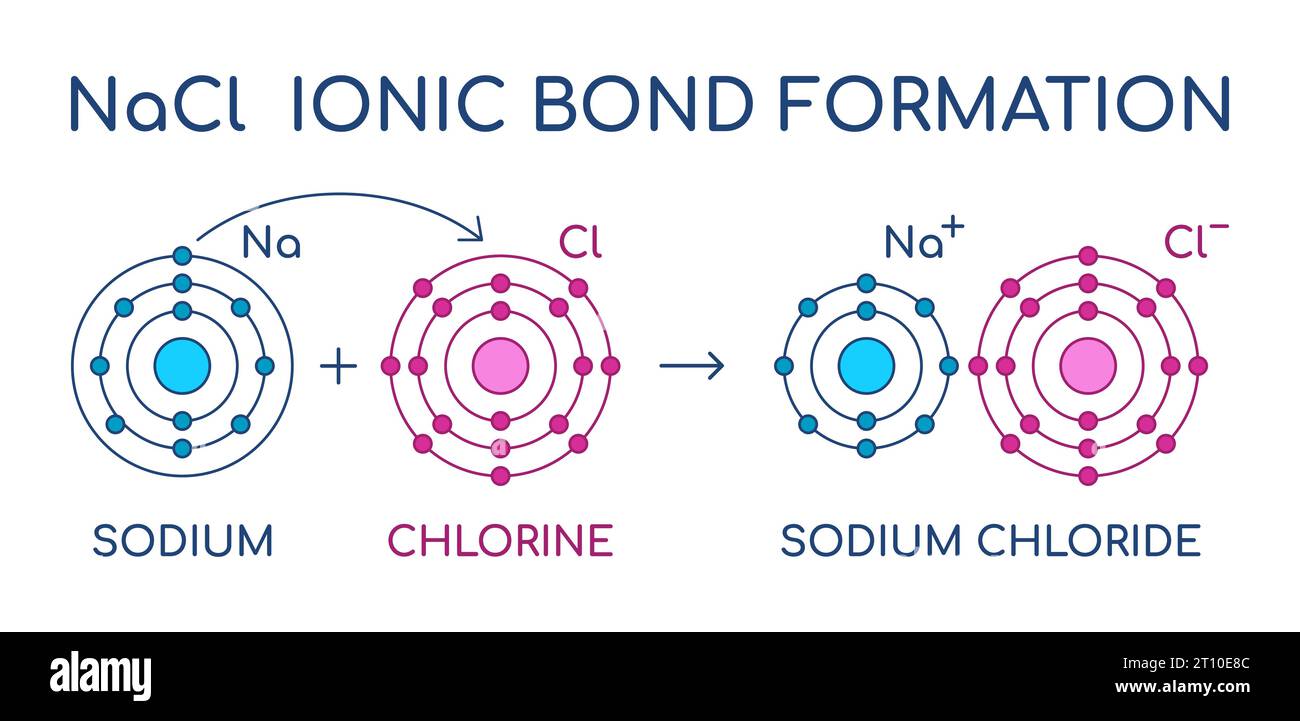
Sodium Chloride ionic bond formation. NaCl structure. Sodium and
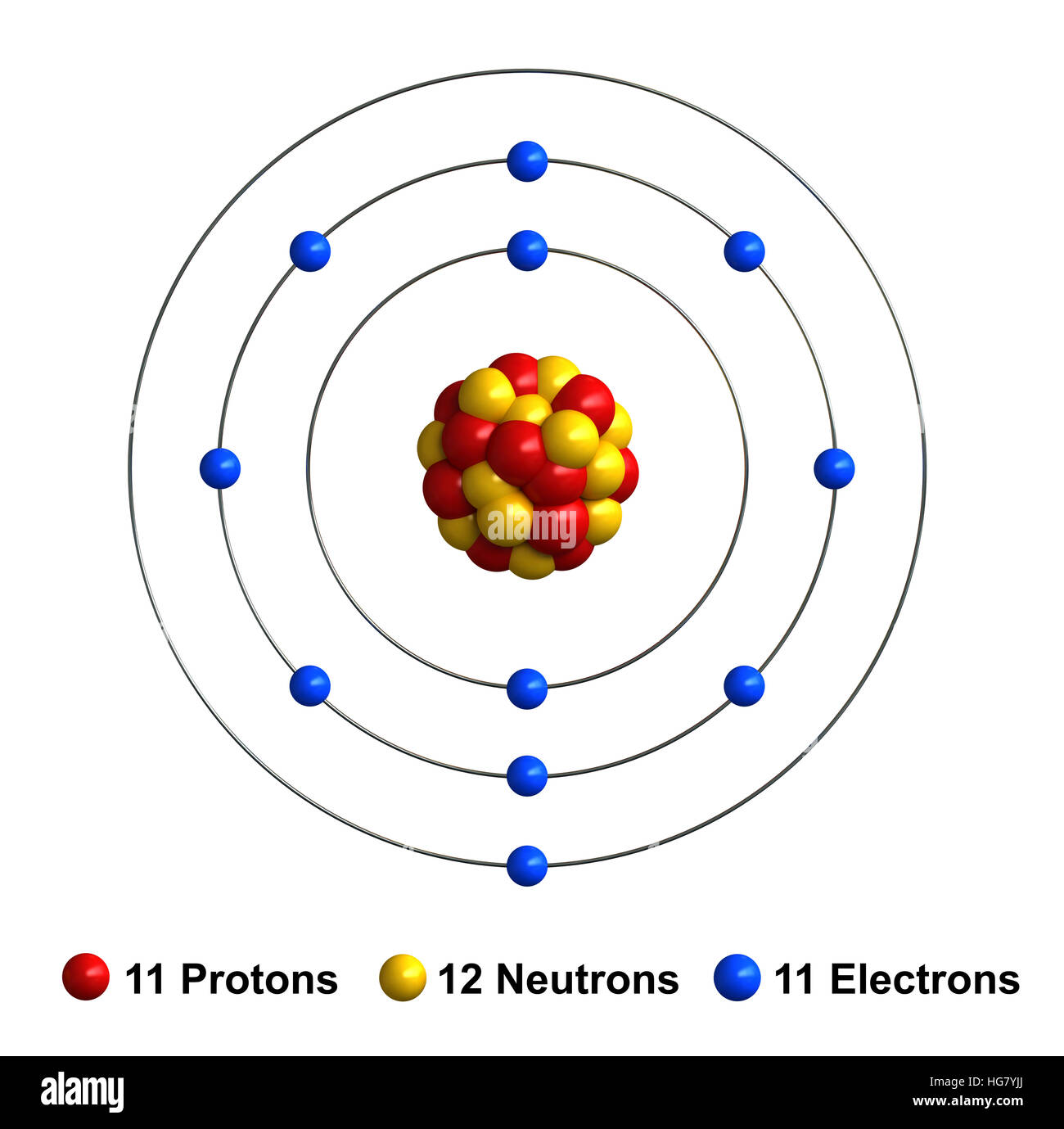
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. (i) formation of sodium ion: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A neutral sodium atom consists of 11 protons and 11 electrons.
Sodium Atom Bohr Model Vector Illustration 267662272
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. A neutral sodium atom consists of 11 protons and 11 electrons. (i) formation of sodium ion: When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative.
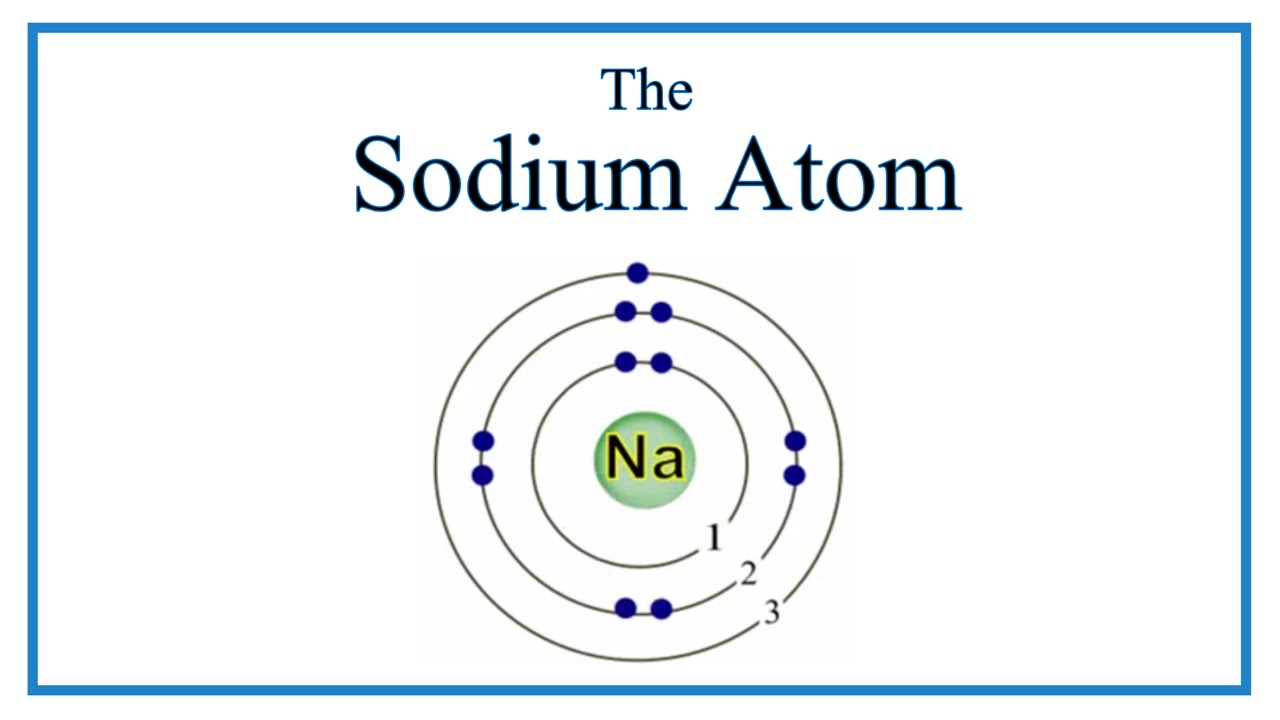
Draw a diagram to show the electronic structure of a sodium Quizlet
The sodium ion, na +, has the electron configuration with an octet of electrons. (i) formation of sodium ion: Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium loses its outermost electron, the atom transforms. A neutral sodium atom consists of 11 protons and 11 electrons.
When A Sodium Atom Reacts With A Chlorine Atom To Form A Compound The
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When sodium loses its outermost electron, the atom transforms. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation.
The structure of the sodium atom is shown in the figure given alongside
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. (i) formation of sodium ion: A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge.
Solved Two students worked together to create a bohr model of a sodium
When sodium loses its outermost electron, the atom transforms. (i) formation of sodium ion: The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative.
How To Draw A Sodium Ion Image to u
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. When sodium loses its outermost electron, the atom transforms. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons. A neutral.
Sodium Ion Bohr Model
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. The sodium ion, na +, has the electron configuration with an octet of electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative. When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+.
Ions Form When Atoms Lose Or Gain Electrons Close Electron Subatomic Particle, With A Negative.
When sodium atoms form ions, they always form a 1+ charge, never a 2+ or 3+ or even 1− charge. A neutral sodium atom consists of 11 protons and 11 electrons. When sodium loses its outermost electron, the atom transforms. (i) formation of sodium ion:
The Sodium Ion, Na +, Has The Electron Configuration With An Octet Of Electrons.
Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative.