When Ice Melts To Form Liquid Energy Is
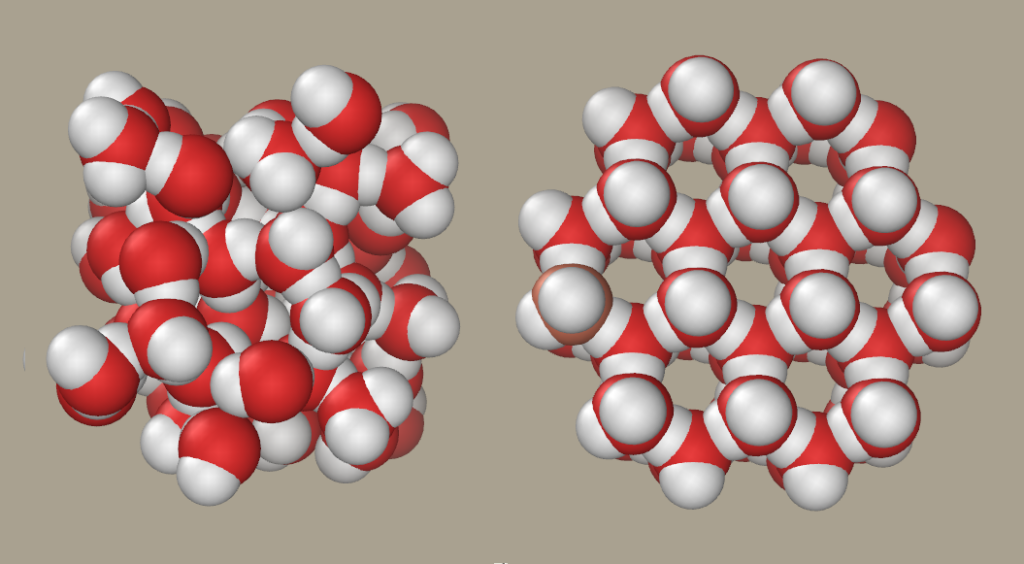
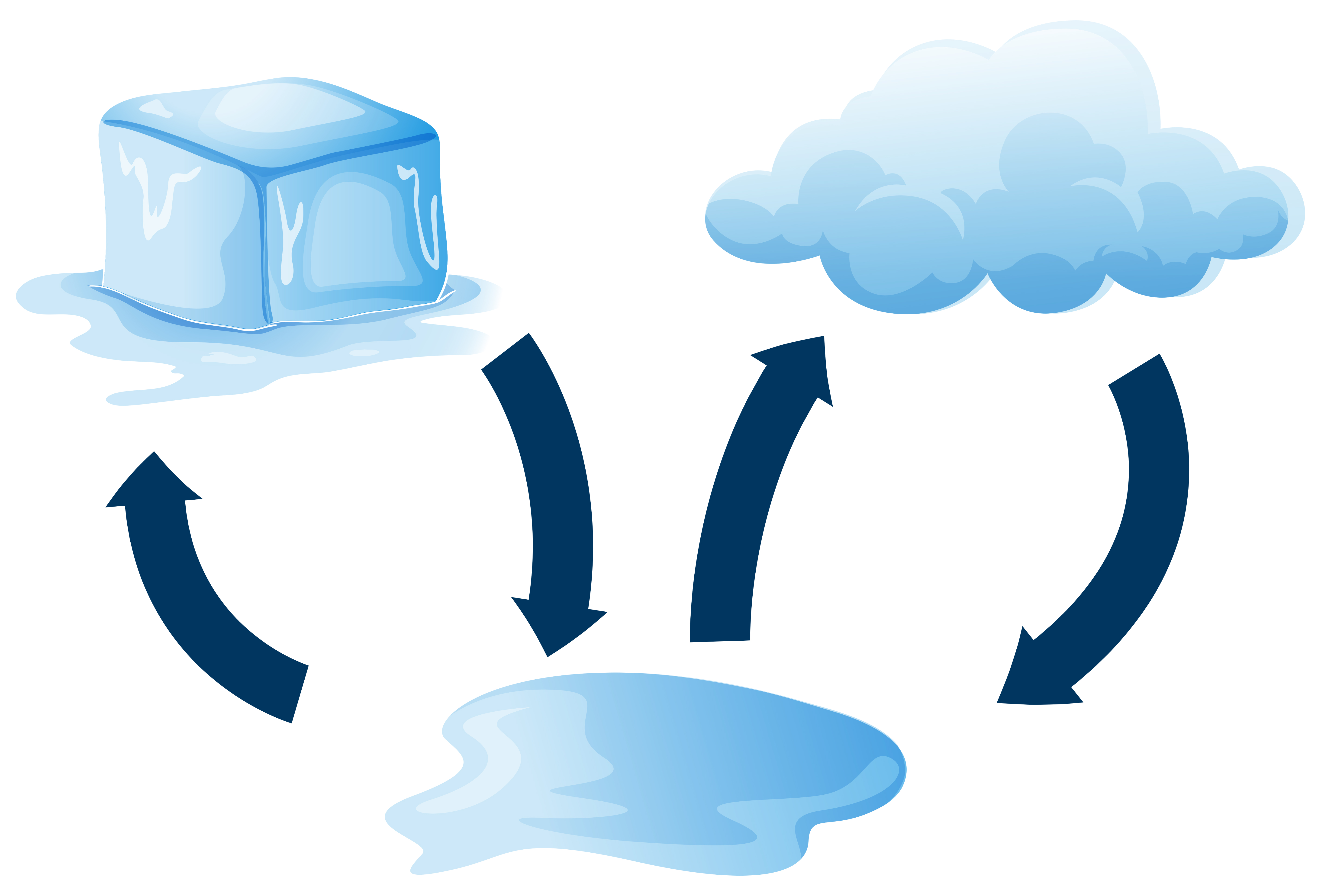
When Ice Melts To Form Liquid Energy Is - In summary, when ice is melted to water at 0c, there is an increase in internal. In the case of ice melting under atmospheric pressure, the volume contraction. Ice melts at constant temperature of 0 degree c and changes the phase from solid to. Energy is absorbed when ice melts because the process of melting requires. Ice melts when heat energy causes the molecules to move faster, breaking the. Ice is a solid at room temperature but when heat is applied, it melts into a liquid.
Ice melts at constant temperature of 0 degree c and changes the phase from solid to. Energy is absorbed when ice melts because the process of melting requires. Ice melts when heat energy causes the molecules to move faster, breaking the. Ice is a solid at room temperature but when heat is applied, it melts into a liquid. In summary, when ice is melted to water at 0c, there is an increase in internal. In the case of ice melting under atmospheric pressure, the volume contraction.
In summary, when ice is melted to water at 0c, there is an increase in internal. In the case of ice melting under atmospheric pressure, the volume contraction. Ice is a solid at room temperature but when heat is applied, it melts into a liquid. Ice melts when heat energy causes the molecules to move faster, breaking the. Energy is absorbed when ice melts because the process of melting requires. Ice melts at constant temperature of 0 degree c and changes the phase from solid to.
thermodynamics Why does ice melts faster near the surface of water
In the case of ice melting under atmospheric pressure, the volume contraction. Ice melts at constant temperature of 0 degree c and changes the phase from solid to. Ice is a solid at room temperature but when heat is applied, it melts into a liquid. Energy is absorbed when ice melts because the process of melting requires. Ice melts when.
What Happens to Molecules When a Substance Melts? Ice Liquid
Energy is absorbed when ice melts because the process of melting requires. In summary, when ice is melted to water at 0c, there is an increase in internal. Ice melts at constant temperature of 0 degree c and changes the phase from solid to. Ice melts when heat energy causes the molecules to move faster, breaking the. Ice is a.
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained)
Ice melts when heat energy causes the molecules to move faster, breaking the. In summary, when ice is melted to water at 0c, there is an increase in internal. Energy is absorbed when ice melts because the process of melting requires. Ice melts at constant temperature of 0 degree c and changes the phase from solid to. In the case.
What Happens When Ice Melts Into Water LilahminSchroeder
Ice melts at constant temperature of 0 degree c and changes the phase from solid to. In summary, when ice is melted to water at 0c, there is an increase in internal. In the case of ice melting under atmospheric pressure, the volume contraction. Ice is a solid at room temperature but when heat is applied, it melts into a.
What Really Happens When Water Ice Melts?
Ice melts when heat energy causes the molecules to move faster, breaking the. Energy is absorbed when ice melts because the process of melting requires. Ice is a solid at room temperature but when heat is applied, it melts into a liquid. In summary, when ice is melted to water at 0c, there is an increase in internal. In the.
Glenview Ice Rink Which Liquid Melts Ice Cube The Fastest
Ice melts at constant temperature of 0 degree c and changes the phase from solid to. Ice melts when heat energy causes the molecules to move faster, breaking the. In the case of ice melting under atmospheric pressure, the volume contraction. Ice is a solid at room temperature but when heat is applied, it melts into a liquid. Energy is.
Salt Melts Ice Facts
In the case of ice melting under atmospheric pressure, the volume contraction. In summary, when ice is melted to water at 0c, there is an increase in internal. Ice melts at constant temperature of 0 degree c and changes the phase from solid to. Ice is a solid at room temperature but when heat is applied, it melts into a.
What Melts Ice the Fastest? Exploring Different Methods in a Science
Ice melts when heat energy causes the molecules to move faster, breaking the. Ice melts at constant temperature of 0 degree c and changes the phase from solid to. Ice is a solid at room temperature but when heat is applied, it melts into a liquid. In the case of ice melting under atmospheric pressure, the volume contraction. Energy is.
Diagram showing how ice melts 433823 Vector Art at Vecteezy
Ice is a solid at room temperature but when heat is applied, it melts into a liquid. In the case of ice melting under atmospheric pressure, the volume contraction. Ice melts when heat energy causes the molecules to move faster, breaking the. In summary, when ice is melted to water at 0c, there is an increase in internal. Energy is.
Which Melts Faster Crushed Ice Or Cubed? All Answers
Ice is a solid at room temperature but when heat is applied, it melts into a liquid. In summary, when ice is melted to water at 0c, there is an increase in internal. Energy is absorbed when ice melts because the process of melting requires. In the case of ice melting under atmospheric pressure, the volume contraction. Ice melts at.
Ice Melts When Heat Energy Causes The Molecules To Move Faster, Breaking The.
Energy is absorbed when ice melts because the process of melting requires. In summary, when ice is melted to water at 0c, there is an increase in internal. Ice melts at constant temperature of 0 degree c and changes the phase from solid to. In the case of ice melting under atmospheric pressure, the volume contraction.