What Type Of Electron Is Available To Form Bonds
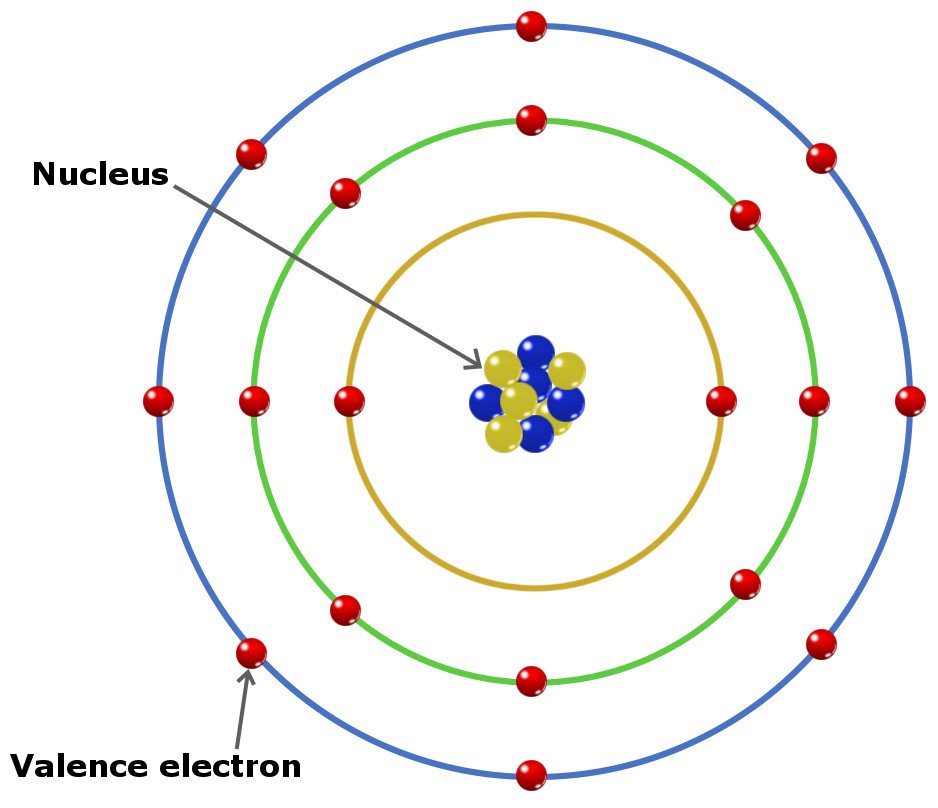
What Type Of Electron Is Available To Form Bonds - Atoms can join together by forming a chemical bond, which is a very strong attraction between. Valence electrons, which are the electrons in the outermost energy level of an. The type of electron available to form bonds is the valence electron. Ionic bonds, which involve a transfer of. In conclusion, the type of electron available to form bonds is the valence electron. Two main types of bonds can be formed:
Two main types of bonds can be formed: Valence electrons, which are the electrons in the outermost energy level of an. In conclusion, the type of electron available to form bonds is the valence electron. The type of electron available to form bonds is the valence electron. Ionic bonds, which involve a transfer of. Atoms can join together by forming a chemical bond, which is a very strong attraction between.
Ionic bonds, which involve a transfer of. In conclusion, the type of electron available to form bonds is the valence electron. Two main types of bonds can be formed: Valence electrons, which are the electrons in the outermost energy level of an. Atoms can join together by forming a chemical bond, which is a very strong attraction between. The type of electron available to form bonds is the valence electron.
Valence Electron How To Discuss
Two main types of bonds can be formed: Ionic bonds, which involve a transfer of. The type of electron available to form bonds is the valence electron. In conclusion, the type of electron available to form bonds is the valence electron. Atoms can join together by forming a chemical bond, which is a very strong attraction between.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
The type of electron available to form bonds is the valence electron. Ionic bonds, which involve a transfer of. Atoms can join together by forming a chemical bond, which is a very strong attraction between. In conclusion, the type of electron available to form bonds is the valence electron. Two main types of bonds can be formed:
Electron Configuration Of Oxygen In Ground State
The type of electron available to form bonds is the valence electron. Two main types of bonds can be formed: Atoms can join together by forming a chemical bond, which is a very strong attraction between. Valence electrons, which are the electrons in the outermost energy level of an. In conclusion, the type of electron available to form bonds is.
What type of electron is available to form bonds?
Valence electrons, which are the electrons in the outermost energy level of an. Atoms can join together by forming a chemical bond, which is a very strong attraction between. In conclusion, the type of electron available to form bonds is the valence electron. Two main types of bonds can be formed: The type of electron available to form bonds is.
How To Form Ionic Bonds
Atoms can join together by forming a chemical bond, which is a very strong attraction between. Two main types of bonds can be formed: In conclusion, the type of electron available to form bonds is the valence electron. Ionic bonds, which involve a transfer of. The type of electron available to form bonds is the valence electron.
Types of Chemical Bonds
Ionic bonds, which involve a transfer of. The type of electron available to form bonds is the valence electron. Two main types of bonds can be formed: Valence electrons, which are the electrons in the outermost energy level of an. Atoms can join together by forming a chemical bond, which is a very strong attraction between.
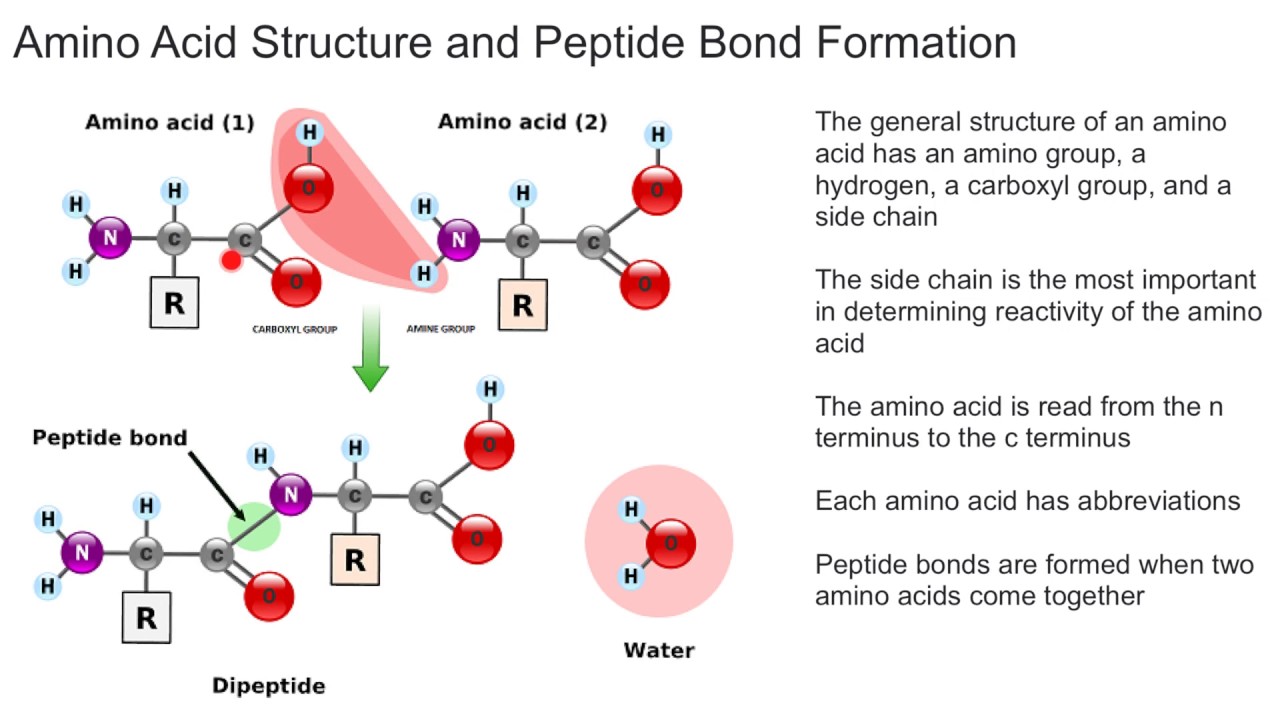
What Do Peptide Bonds Do
The type of electron available to form bonds is the valence electron. Atoms can join together by forming a chemical bond, which is a very strong attraction between. Valence electrons, which are the electrons in the outermost energy level of an. Ionic bonds, which involve a transfer of. In conclusion, the type of electron available to form bonds is the.
Electron pair chemistry Britannica
Valence electrons, which are the electrons in the outermost energy level of an. Ionic bonds, which involve a transfer of. In conclusion, the type of electron available to form bonds is the valence electron. Two main types of bonds can be formed: Atoms can join together by forming a chemical bond, which is a very strong attraction between.
What are the 4 types bonds? Leia aqui What are the 4 types of bonds
In conclusion, the type of electron available to form bonds is the valence electron. Ionic bonds, which involve a transfer of. Atoms can join together by forming a chemical bond, which is a very strong attraction between. Valence electrons, which are the electrons in the outermost energy level of an. Two main types of bonds can be formed:
Chemical Bonds Anatomy and Physiology I
In conclusion, the type of electron available to form bonds is the valence electron. Valence electrons, which are the electrons in the outermost energy level of an. Two main types of bonds can be formed: The type of electron available to form bonds is the valence electron. Atoms can join together by forming a chemical bond, which is a very.
Ionic Bonds, Which Involve A Transfer Of.
Two main types of bonds can be formed: The type of electron available to form bonds is the valence electron. In conclusion, the type of electron available to form bonds is the valence electron. Valence electrons, which are the electrons in the outermost energy level of an.