In Forming A Solution The Solute Always
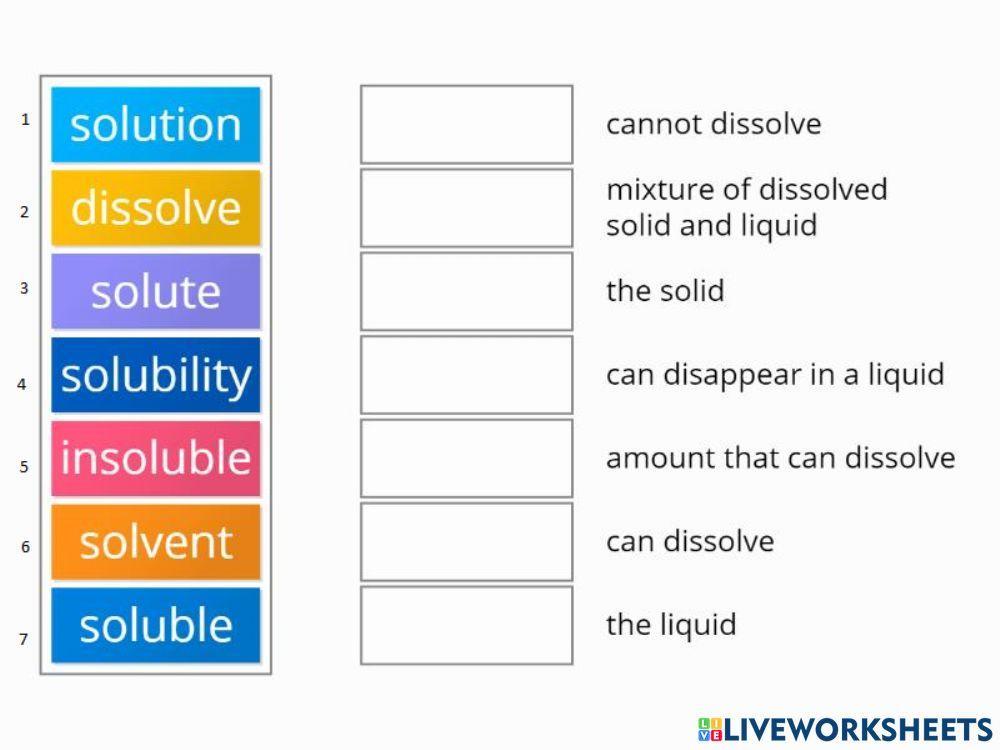
In Forming A Solution The Solute Always - What causes a solution to form? A strong electrolyte always forms a very concentrated solution and a weak. The simple answer is that the solvent and the. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. In forming a solution, the solute always: Dissolves dissociates both dissolves and dissociates.
In order to form a solution, the solute must be surrounded, or solvated, by the solvent. A strong electrolyte always forms a very concentrated solution and a weak. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. In forming a solution, the solute always: What causes a solution to form? The simple answer is that the solvent and the. Dissolves dissociates both dissolves and dissociates.
In order to form a solution, the solute must be surrounded, or solvated, by the solvent. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form? Dissolves dissociates both dissolves and dissociates. In forming a solution, the solute always: The simple answer is that the solvent and the. A strong electrolyte always forms a very concentrated solution and a weak.
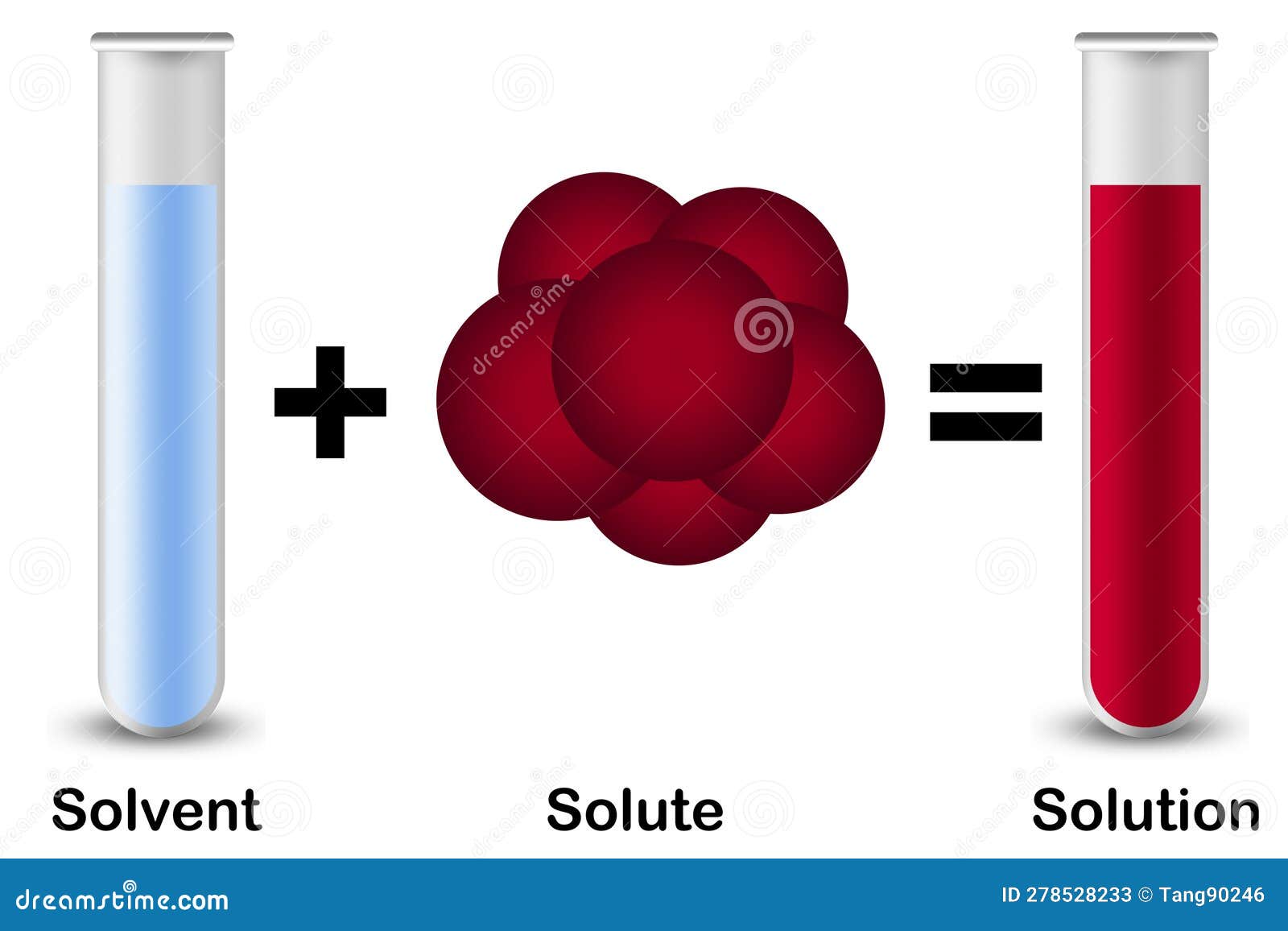
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples
When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form? Dissolves dissociates both dissolves and dissociates. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. The simple answer is that the solvent and the.
Examples Of Solute, Solvent And Solution Examples Of Solute, 44 OFF
When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. In forming a solution, the solute always: Dissolves dissociates both dissolves and dissociates. A strong electrolyte always forms a very concentrated solution and a weak. What causes a solution to form?
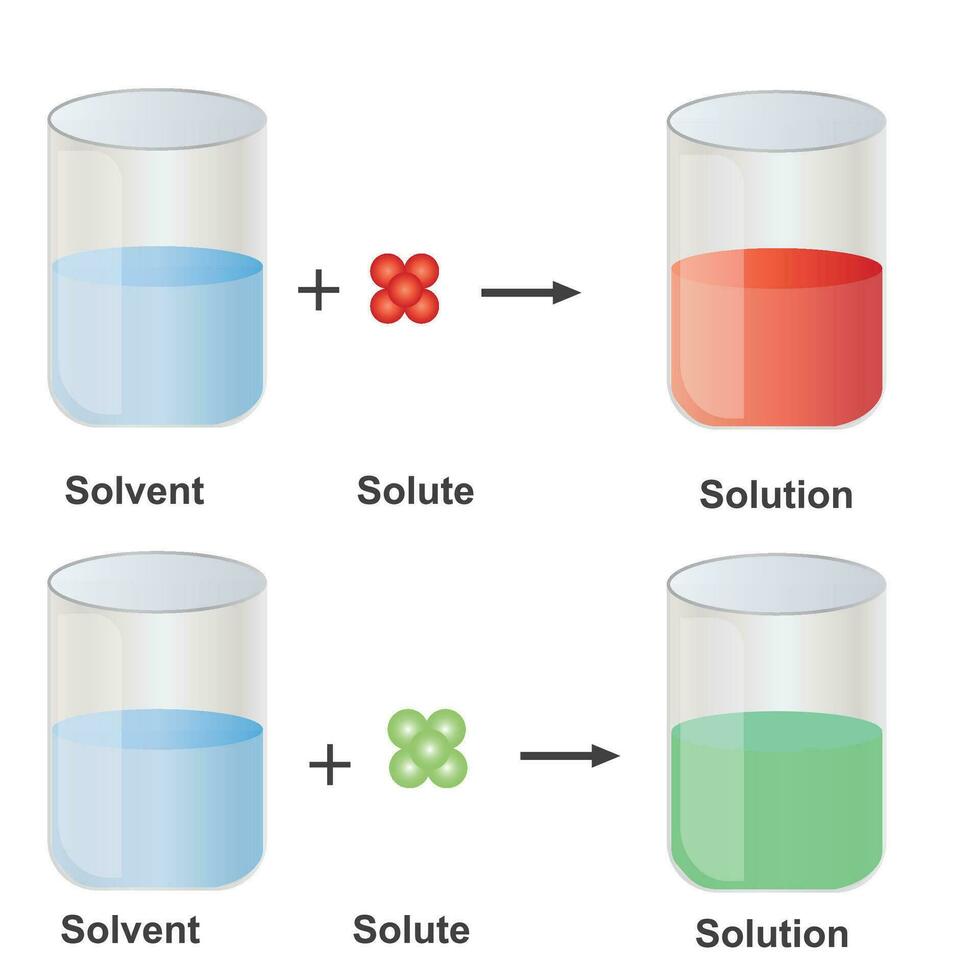
Understanding the Solute and Solvent Relationship
A strong electrolyte always forms a very concentrated solution and a weak. In forming a solution, the solute always: In order to form a solution, the solute must be surrounded, or solvated, by the solvent. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form?
In a solution, is the solute always a solid.
What causes a solution to form? When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. A strong electrolyte always forms a very concentrated solution and a weak. The simple answer is that the solvent and the. In forming a solution, the solute always:
Solute In Science
Dissolves dissociates both dissolves and dissociates. A strong electrolyte always forms a very concentrated solution and a weak. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. The simple answer is that the solvent and the.
Solutions. Solubility homogeneous mixture. Solute, solvent and solution
The simple answer is that the solvent and the. A strong electrolyte always forms a very concentrated solution and a weak. In forming a solution, the solute always: Dissolves dissociates both dissolves and dissociates. In order to form a solution, the solute must be surrounded, or solvated, by the solvent.
Solute Energy Education
A strong electrolyte always forms a very concentrated solution and a weak. In forming a solution, the solute always: When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. What causes a solution to form? In order to form a solution, the solute must be surrounded, or solvated, by the solvent.
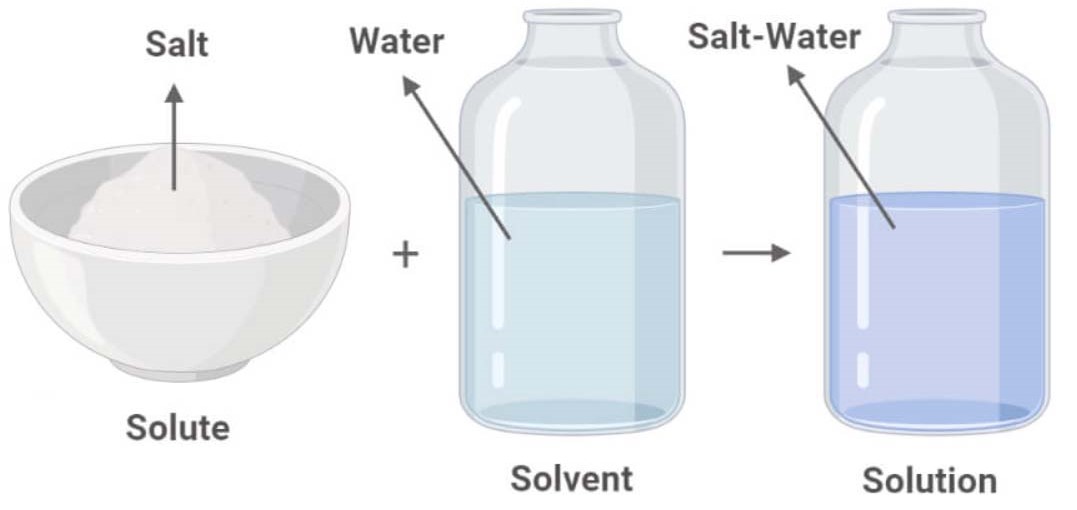
Solute, Solvent and Solution Isolated with Red Solute Stock
In forming a solution, the solute always: A strong electrolyte always forms a very concentrated solution and a weak. When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. The simple answer is that the solvent and the. Dissolves dissociates both dissolves and dissociates.
Solute and Solvent Combinations — Overview & Examples Expii
When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. What causes a solution to form? A strong electrolyte always forms a very concentrated solution and a weak. In forming a solution, the solute always:
Difference Between Solvent and Solute Definition, Properties, Examples
In forming a solution, the solute always: When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. Dissolves dissociates both dissolves and dissociates. In order to form a solution, the solute must be surrounded, or solvated, by the solvent. The simple answer is that the solvent and the.
A Strong Electrolyte Always Forms A Very Concentrated Solution And A Weak.
What causes a solution to form? When a solute dissolves in a solvent, its particles separate and disperse uniformly throughout the. The simple answer is that the solvent and the. Dissolves dissociates both dissolves and dissociates.
In Forming A Solution, The Solute Always:
In order to form a solution, the solute must be surrounded, or solvated, by the solvent.