An Ionic Bond Forms Between A
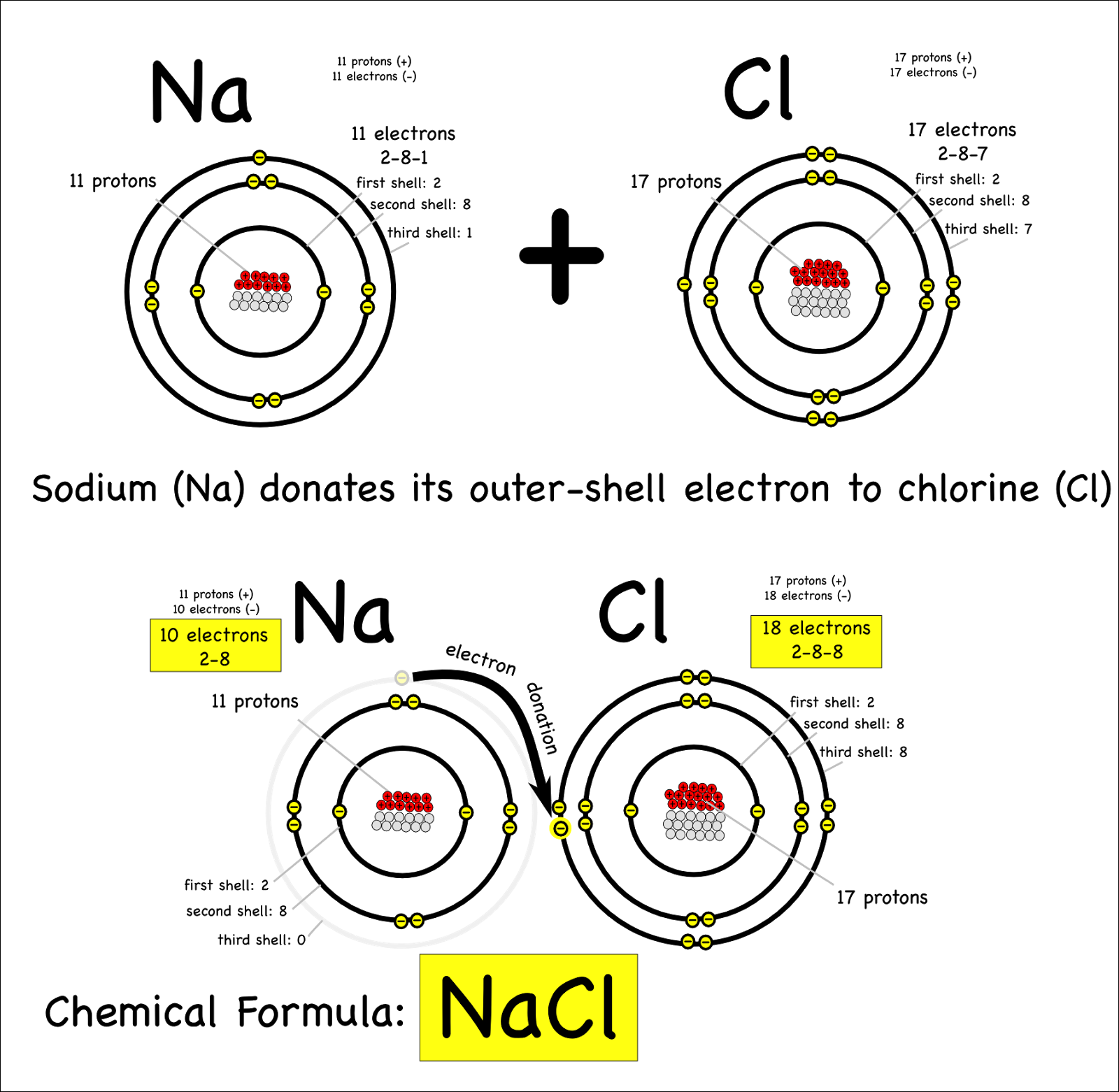
An Ionic Bond Forms Between A - An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s) between atoms. Ionic bonds are formed between cations and anions.
In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative.
A cation is formed when a metal ion. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative.
Diagram Ionic Bond
Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s).
ionic bond Definition, Properties, Examples, & Facts Britannica
Ionic bonding is the complete transfer of valence electron(s) between atoms. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process.
Diagram Of Ionic Bonding
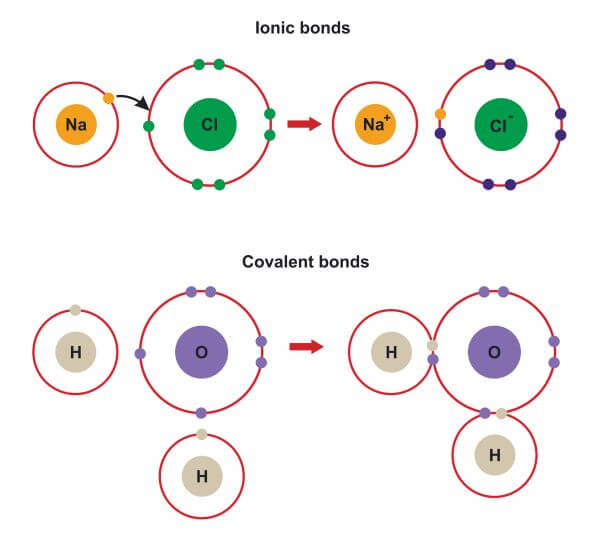
Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations.
Ionic Bonding Diagram
In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s).
Ionic Bond Facts, Definition, Properties, Examples,, 55 OFF
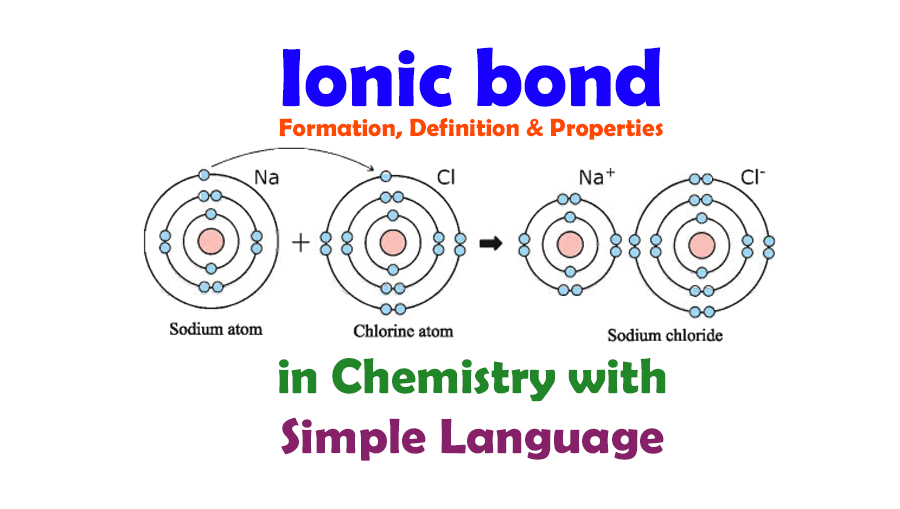
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonding is the complete transfer of valence electron(s) between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonds are formed between cations.
Ionic Bond Examples Biology Dictionary
In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonding is the complete transfer of valence electron(s) between atoms. Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with.
Ionic Bond and Ionic Bond Formation, Definition, Properties in
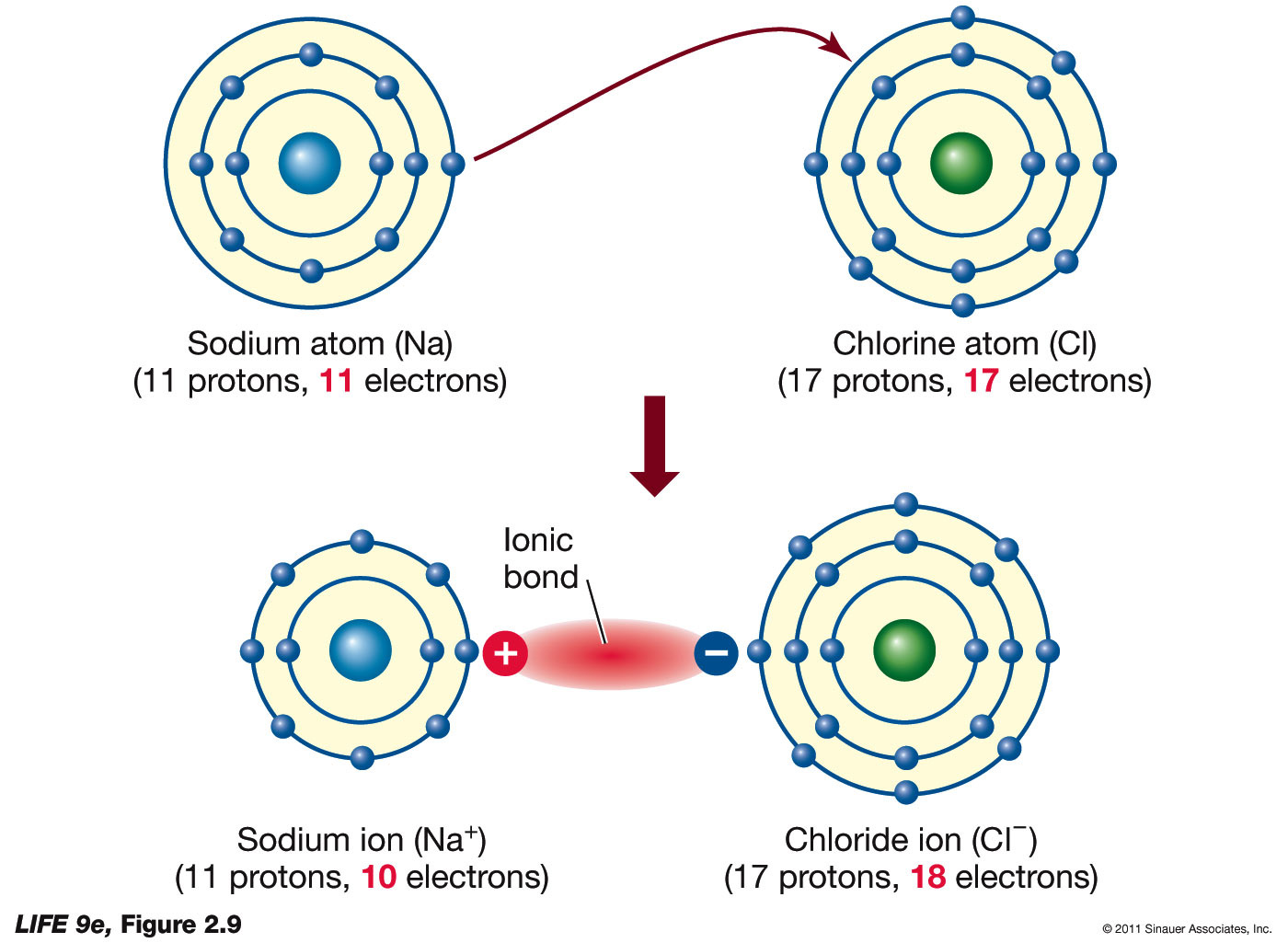
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s).
Examples of Ionic Bonds and Compounds
In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonds are formed between cations and anions. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic bonding is the complete transfer of valence electron(s) between atoms. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. Ionic bonds are formed between cations.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
In an ionic bond, a complete transfer of electrons takes place in the process of bond. A cation is formed when a metal ion. An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative. Ionic bonds are formed between cations and anions. Ionic bonding is the complete transfer of valence electron(s).
An Ionic Bond Forms Between A ____ Ion With A Positive Charge And A ____ Ion With A Negative.
Ionic bonds are formed between cations and anions. In an ionic bond, a complete transfer of electrons takes place in the process of bond. Ionic bonding is the complete transfer of valence electron(s) between atoms. A cation is formed when a metal ion.
.PNG)